A (More) Physically Based First Approximation
for Electron Probe Quantification
(Presented at MAS, August 1999, Portland, OR)
By John J. Donovan*, Nicholas E. Pingitore Jr.**, and Raymond Jeanloz*
*Department of Geology and Geophysics, The University of California, Berkeley, California 94720-4767
**Department of Geological Sciences, The University of Texas at El Paso, El Paso, Texas 79968-0555
- Experimental: Isotope measurements vs. the mass-intensity assumption in EPMA
- Electron fractions: a first approximation based on interactions between incident electrons and orbital electron and nuclear protons (no interaction with neutrons)
- Justification: Monte-Carlo calculations and empirical k-ratio datasets
- Quantification: A first step towards a truly physically based quantitative model
This morning we would like to present some recent measurements that seem to suggest that some of our assumptions in electron probe microanalysis (EPMA) are perhaps not as well founded they might be. Following this, we will propose, and attempt to justify, the use of a first approximation based solely on atomic number. But primarily we will be showing that much more work is needed before we have a truly physically based model for quantitatively correcting x-ray intensities in EPMA.
First, I would like to start with the question of mass: what role does mass, that is atomic mass, play in electron probe microanalysis?

"...if all the elements be arranged in order of their atomic weights, a periodic repetition of properties is obtained."
"A few atomic weights will probably require correction; for example Te cannot have the atomic weight 128, but rather 123-126.
- Mendeleyev
Historically, mass was one of the earliest properties of matter that was measured with any significant precision, and is what Mendeleyev and other investigators in the 19th century, used as the basis for organizing the first periodic tables...
The ordering of the periodic table of elements, by atomic mass (some using very imaginative layouts) may seem amusing to us today. But, the assumption worked well enough for Mendeleyev to eventually predict several new elements. And after he noticed that a few elements appeared to be out of place in his new table, rather than discard his model, he made the bold prediction that the measurements of those atomic weights were in error. Mendeleyev turned out to be, in fact, correct more often than not, but in some cases, for example, the element Te, he was mistaken.
[periodic table]
The reason being that the atomic weight of tellurium is actually greater than that of iodine, the next element in the periodic table. This interesting juxtaposition of atomic mass and atomic number occurs in two other places in the periodic table, between cobalt and nickel, here, and between argon and potassium here.
I’ll explain in moment why this might be significant to us today, but in the meantime it suffices to say that it wasn’t until the development of atomic theory and of the concept of an atomic number at the end of the 19th century, that the periodic table really started to make sense for both chemistry and physics.
But back to EPMA, where now, over 100 years later, as incredible as it may seem, we appear to have made the same mistake as made by Mendeleyev, that is, in confusing a mass effect with an atomic number effect.
Castaing’s Empirical Observation "first approximation"
![]()
where :
and
are mass fractions in the sample and standard
and
are x-ray intensities of a specific transition measured in the sample and the standard
Now as you have heard so much this week, some fifty years ago, a model was proposed by Raymond Castaing that attempted to predict the generation of x-ray intensities from electron bombardment of solid samples. He found that the characteristic x-ray intensity of an element emitted from a compound, relative to the intensity emitted from the pure element, seemed to be correlated to the mass fraction of the element in the compound.
It was an amazing accomplishment. In fact, it was so successful in it’s ability to predict composition, that it immediately inspired many scientists, to extend, refine and improve that original hypothesis by means of various adjustments and corrections, so that today, we can typically achieve accuracies of as little as a few percent in a wide variety of compounds. So what could possibly be wrong with the model?
Well, what exactly is a model? One part of the answer is that a model can make predictions that can be tested, and it would appear that Castaing’s model is fairly successful at meeting this criteria. But a scientific model must do more than just merely predict, it must be able to demonstrate a "causal" relationship.
Now the following thought has probably occurred to all of you at one time or another- characteristic fluorescent x-rays are produced by the interaction of primary beam electrons with orbital electrons (that is atomic number), so why, is not only Castaing’s approximation based on mass fraction, but also various other terms in current ZAF and phi-rho-z algorithms?
It is true, for example, that in the case of calculating average absorption in compounds, we calculate the average mass absorption coefficient by the use of the mass fractions, but of course, that is simply because mass absorption coefficients are exactly that- mass absorption coefficients. They are linear absorption coefficients that have been normalized to mass. But other situations are less easily justified.
Assumed mass fraction scaling in electron-solid interactions for average backscatter loss factor, R, in compounds
![]()
- Duncumb and Reed (1968)
For instance it is often assumed that the average backscatter in a compound can be modeled based on the sum of some fraction of the pure element backscatter yields. Castaing and many others since then, have assumed mass fraction scaling for these processes, but is that reasonable?
"....any law according to which emitted x-ray intensities should be proportional to mass fractions, cannot have solid physical foundations."
- K. F. J. Heinrich, 1981 in "Electron Probe Quantitation"
Almost 20 years ago, Kurt Heinrich suggested that "emitted x-ray intensities" cannot "be proportional to mass fractions". We believe that he is correct. But what evidence is there to refute the assumption of mass scaling in EPMA, as Heinrich suggests?
We decided that a conclusive experiment was needed to test the mass hypothesis. As we all know, an atom consists of electrons, protons and neutrons. The electrons are, by mass at least, negligible. It’s the protons plus neutrons that essentially determine atomic mass for an element.
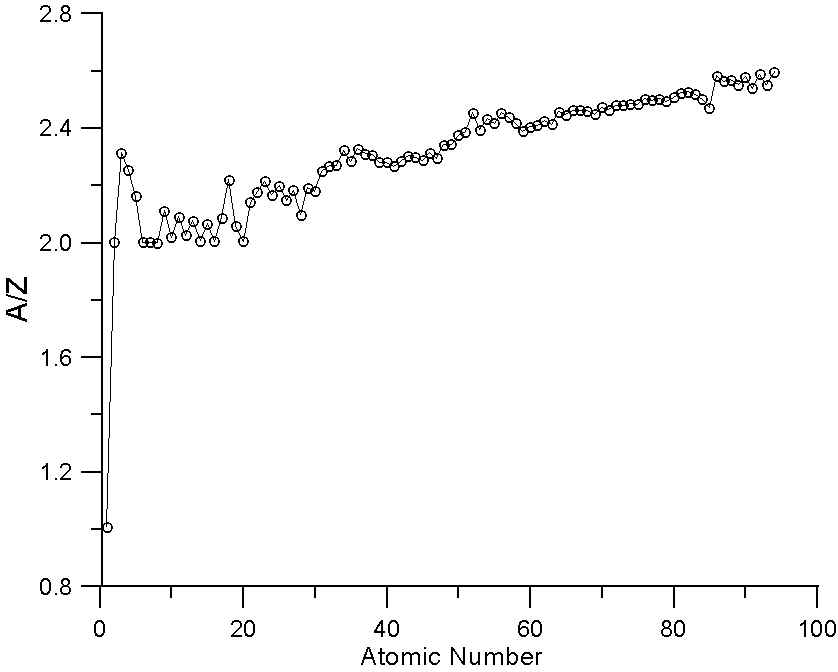
As many investigators have noted, the ratio A/Z in not a constant. In fact, the variation we see here in a plot of A/Z for the periodic table is essentially the average variation in neutron abundance for the various naturally occurring elements. This variation is due to nucleo-synthesis processes in nearby pre-solar supernova and of course, nuclear stability constraints. Because of these processes, the averaged atomic mass for the natural abundance isotopes is not a smooth function of atomic number.
[periodic table]
In essence, that means that some elements have fewer neutrons that one might expect for their atomic number (for example, Ni, atomic number 28, is actually lighter in atomic mass than Co, atomic number 27) and some elements have more neutrons that one might expect (for example Te, atomic number 52, which is heavier than I, atomic number 53).
In any case, to refute the mass-intensity assumption, ideally it would be best to make a comparison where absolutely there exists only a difference in mass, which would allow us to rule out effects due merely to differences in electron and/or proton nuclear charge. Therefore we decided to compare various electron-solid interactions on two isotopes of the same element (that is, same atomic number, but different masses).
Characteristic X-ray Intensities (Ni Ka, Cu Ka and Mo La)
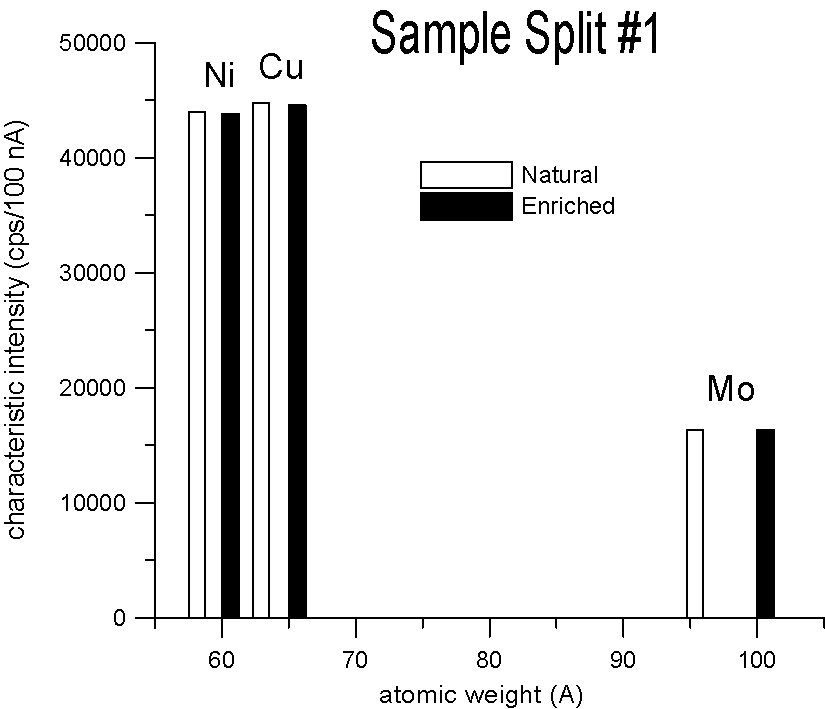
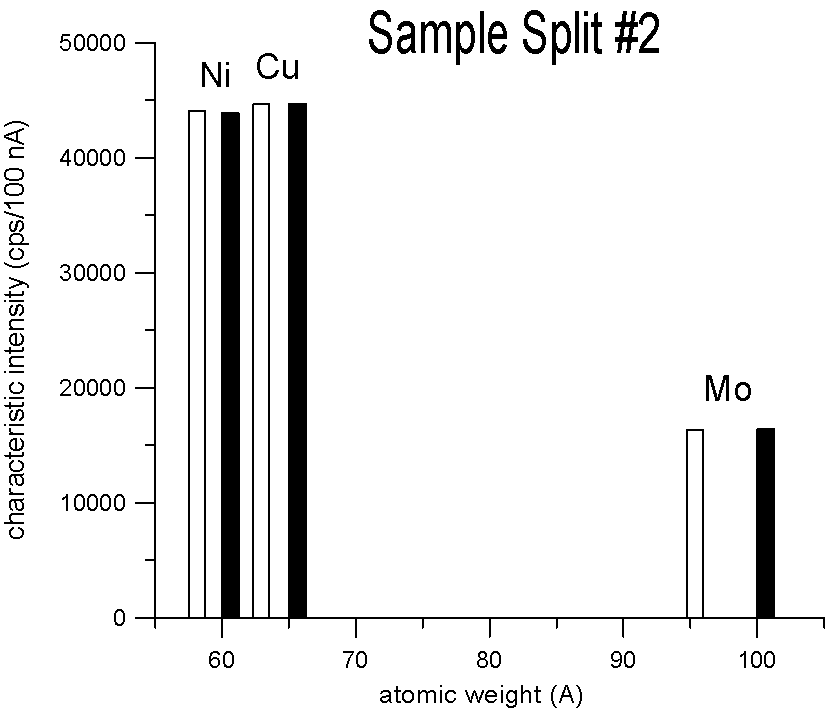
20 KeV, 100 nA, 40 sec., average of 15 points each
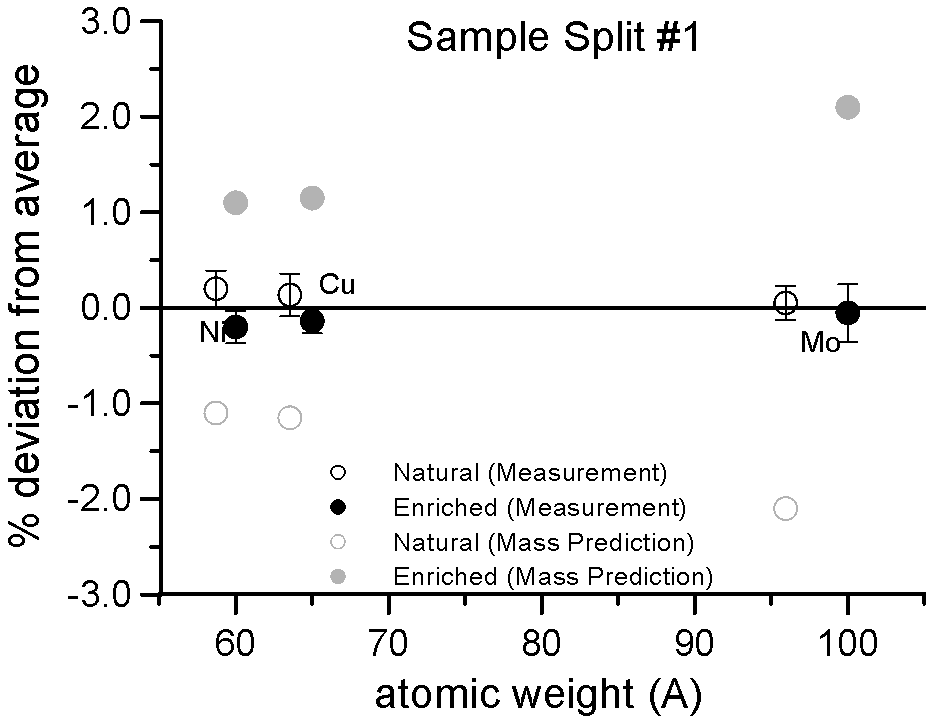
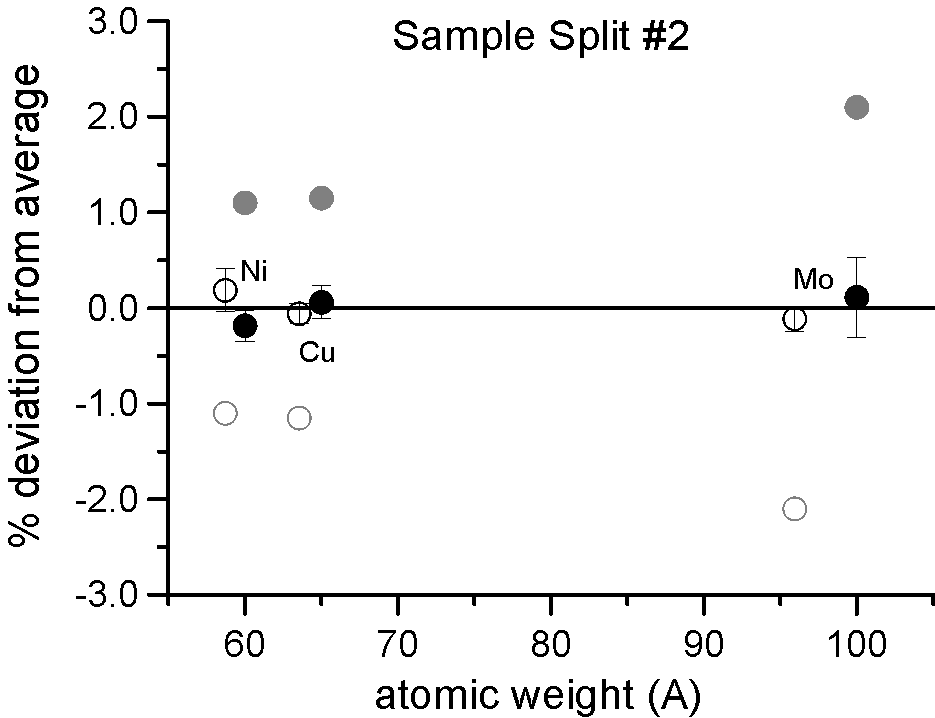
We selected three pairs of elements, for each pair a natural abundance and an enriched stable isotope. Specifically, enriched Ni-60, Cu-65 and Mo-100, which are respectively about 2.2, 2.3 and 4% heavier than their natural abundance siblings. Taken at face value, a mass based first approximation, would seem to predict a significant difference in x-ray generation between the natural and enriched isotope pairs due to their differences in mass.
However, as you can see, replicate measurements on two different splits of the sample, reveal that the emission of characteristic x-ray intensities are similar, at least on this scale.
Characteristic X-ray Intensities (Ni Ka, Cu Ka and Mo La)
20 KeV, 100 nA, 120 sec., Average of 10 points
And in fact, when we take a closer look at the differences between the intensities, it is clearly evident that we can detect no mass effect upon characteristic x-ray production, between the natural and enriched isotope pairs, down to the 0.2% precision level.
Again remember, a mass based first approximation would seemingly predict a 4% difference in the generation and emission of characteristic Mo La x-rays. Here, in a gray color, is the expected x-ray intensity difference for Mo La from a mass based prediction, along that for Ni and Cu here and here.
And even more telling is the fact that if the production of x-rays are unaffected by mass as seen here, then other processes that are also postulated to affect the emitted intensities are apparently not mass dependent either. For example, the emitted x-ray intensity would be expected to be different if the x-ray self-absorption were mass dependent. It is not affected. The electron backscatter loss factor, would also be expected to affect the emitted x-ray intensity if it were mass dependent. It is not affected.
Absorbed Current Measurements on Isotope Pairs
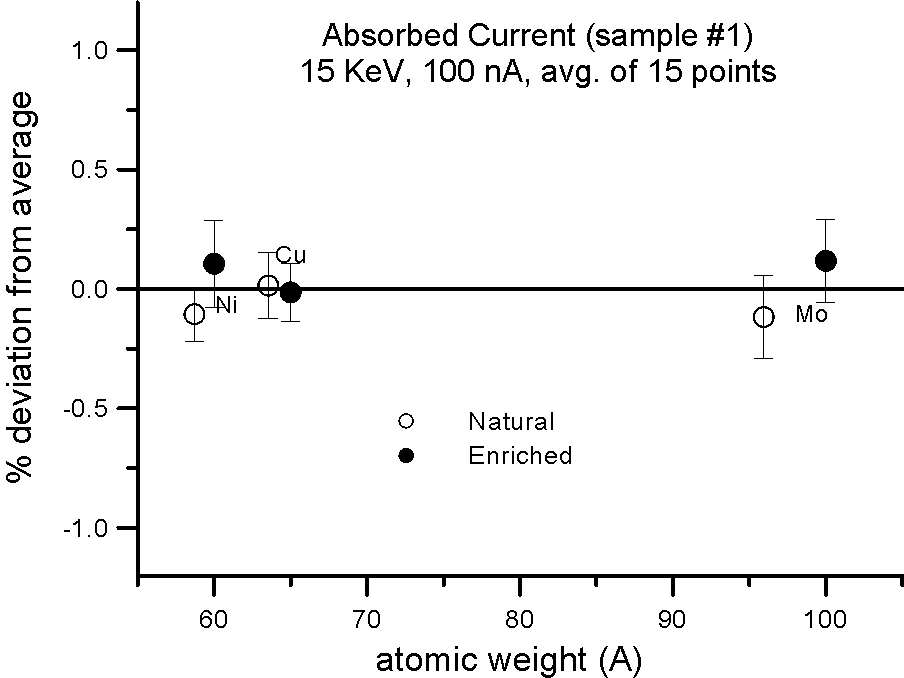
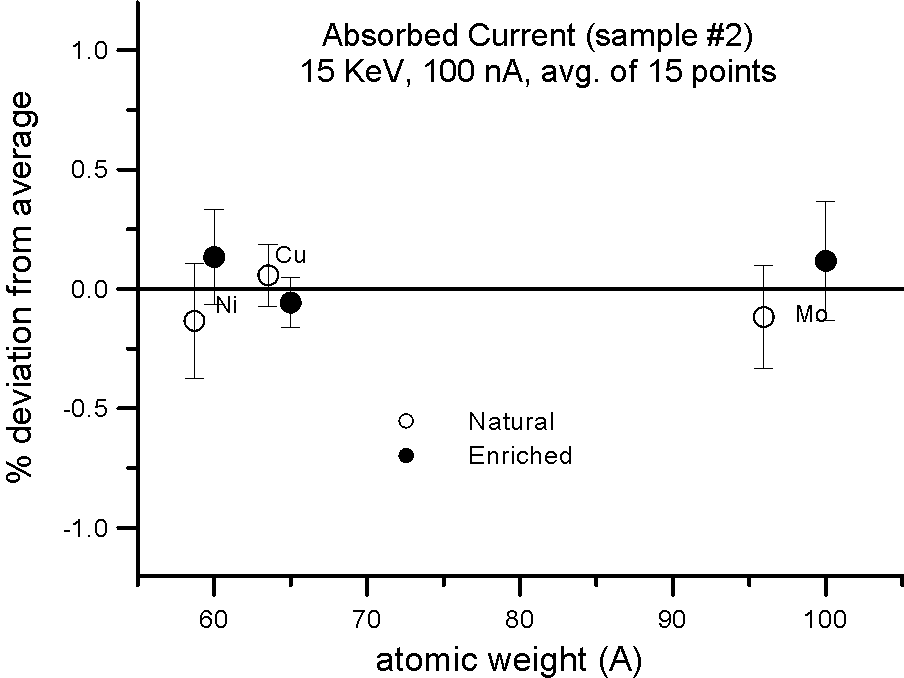
And this is further confirmed by these measurements, where absorbed current was also measured in both sample splits for the isotope pairs. Absorbed current (minus a small fraction due to re-absorbed secondary electrons) is of course reciprocally proportional to the backscatter ratio.
Allow me to emphasize this point again: at EPMA energies and precision levels, electron induced x-ray fluorescence is not influenced by the physical property we call mass!
Why then, this pre-occupation with mass? There is of course, the historical difficulty in trying to predict electron-solid interactions using atom fractions, when it was clearly evident, to all investigators, that mass fractions were by far a better predictor of these phenomena as measured in compounds. Is there another way to describe a fraction of an element in a compound that might be more appropriate for EPMA?
Let’s imagine for a moment that we are an incident electron, zipping along, through a compound of, for example, uranium carbide. That compound has a 1:1 atom ratio of uranium to carbon, so what would we expect the ratio of uranium x-rays or backscattered electrons relative to pure uranium to be?
Well as many similar experiments have shown, it’s certainly not half the contribution from the pure element, so how can it be related to atom fractions? As Castaing astutely noticed, the productions were fairly close (to a first approximation) to the mass fraction of the element.
But we’ve already shown from the isotope data that electron-solid interactions are not appreciably affected by mass, so what scaling model should we use? What does an electron "see" when it travels through a solid material?
Well, it "sees" fields. Electro-magnetic fields, of course, from orbital electrons and from nuclear protons. As we have already shown with the isotope data, it certainly doesn’t interact with neutrons, at least at EPMA energies that we can achieve in the laboratory. But, think again of our uranium carbide example: since uranium has so many more electrons and protons than carbon, the probability of a primary electron interacting with a uranium atom is correspondingly higher than a carbon atom.
In fact, we propose that the interaction ratio (to a first approximation) is exactly that, the "electron" (or proton, if you insist) fraction of the element in a compound.
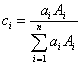
where :
is the atom or atomic fraction of element i in the compound
is the atomic weight of element i in the compound
So, since mass fraction is expressed as the atom-atomic mass fraction, then the electron fraction is simply the atom-atomic number fraction as shown here.
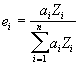
where :
is the atomic fraction of element i in the compound
is the atomic number of element i in the compound
Therefore (again, to a first approximation), essentially, the electron beam penetrates volumes of an equal number of electrons in specimens of different composition. Interestingly enough, Castaing, briefly in his 1960 paper, offered a Z based scaling factor for the generated intensities, only to proceed with the mass based model without explanation.
So why does mass fraction predict x-ray intensities, average backscatter factors and other properties as well as it does? As has been suggested by other investigators, it may simply be because atomic mass is approximately proportional to atomic number.
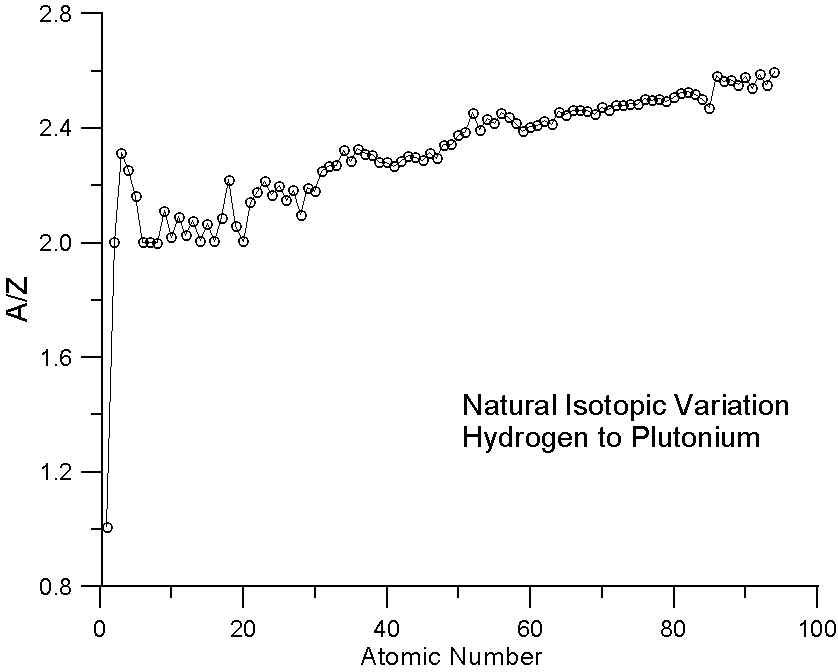
Therefore, we should expect that predictions based on electron fractions would be similar to mass fraction predictions for elements with similar A/Z ratios, but slightly better and in fact particularly better for compounds containing elements that have very different A/Z ratios. In fact, these compounds with very disparate A/Z ratios (that is relatively excessive or deficient numbers of neutrons), will begin to approach the situation we had in our comparison of the natural and enriched isotope pairs. Now, how much of an effect might we expect to see?
Compounds exhibiting small and large differences in A/Z
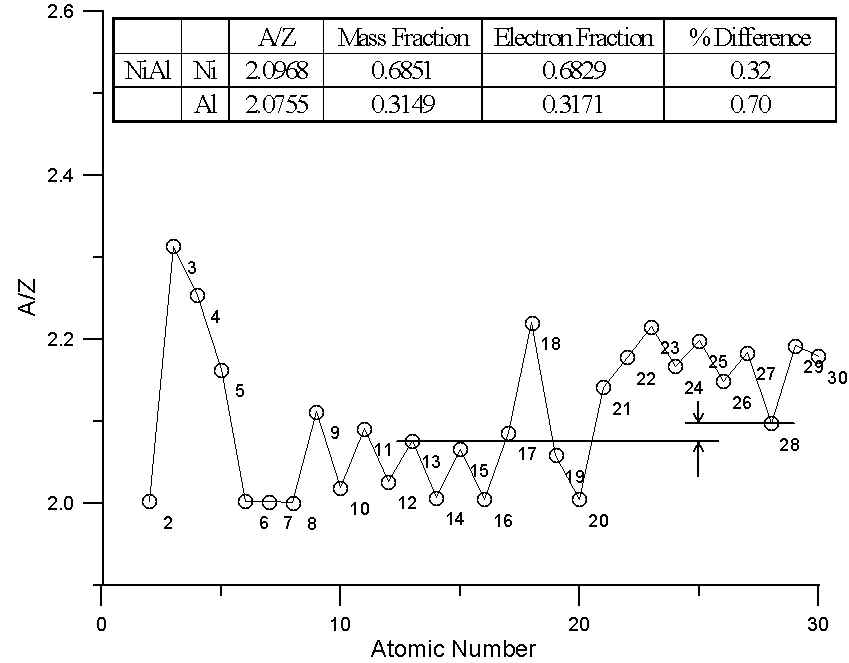
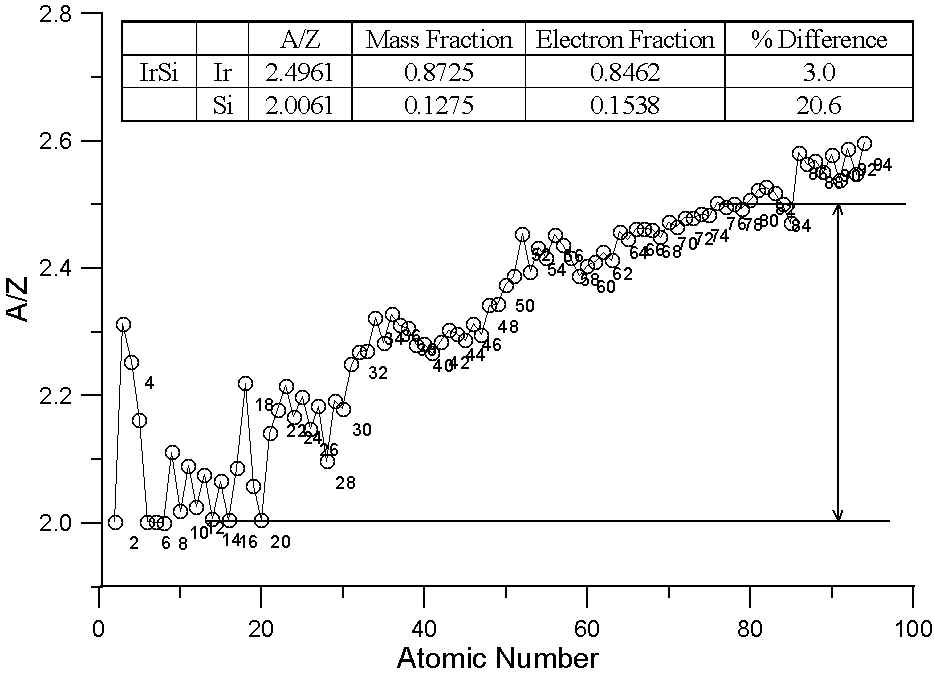
In this plot, we have two different binary compounds, one containing elements with similar A/Z ratios, the other containing elements with large differences in A/Z. In the first case, the difference between the two models is essentially insignificant. Because of the wide variation in A/Z throughout the periodic table, compounds containing elements of quite different atomic number can have similar A/Z ratios.
On the other hand, for the binary compound, IrSi, interestingly enough, which has had problems reported with matrix corrections, the A/Z ratios are quite different and of course, so is the difference between the two models. Over 20% relative difference in the Si concentrations, as you can see!
Now is there any evidence in support of the electron fraction hypothesis? As we know, the generated ratio of x-ray ionizations is not directly measurable, but it can modeled using, of course, monte-carlo!
Monte-Carlo of Generated Intensities for Compounds with Small differences in A/Z
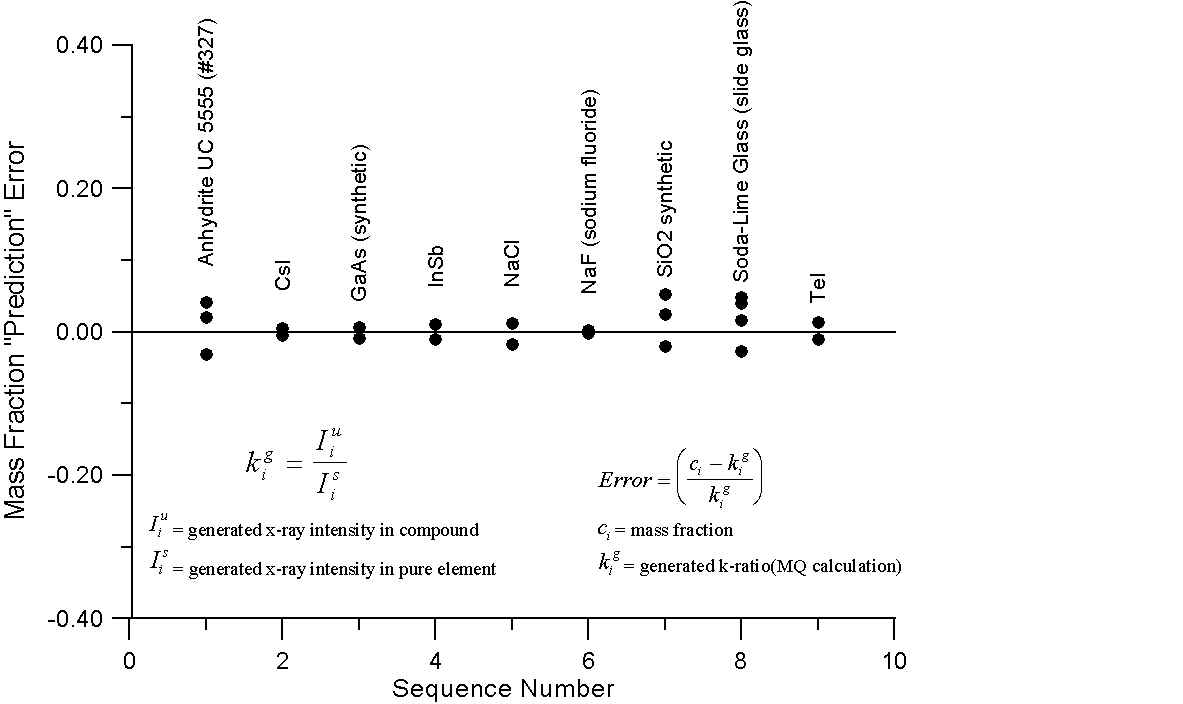
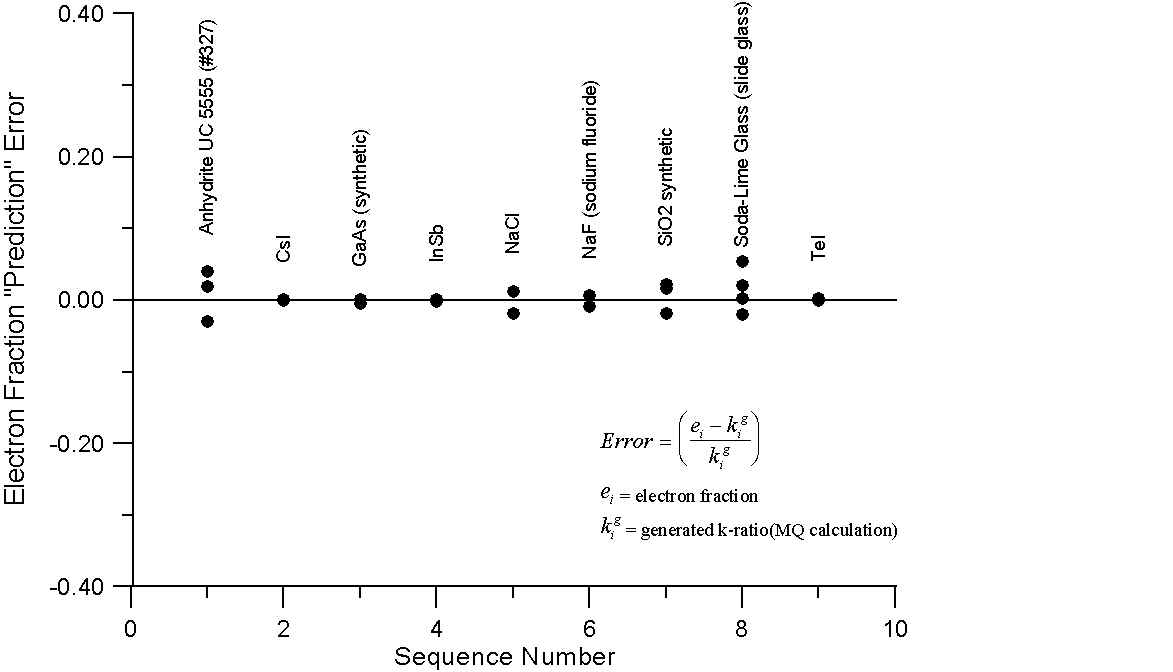
What this graph shows are, for a number of compounds containing elements with little or no difference in A/Z ratios, the errors obtained by comparing the mass fraction and electron fraction of each element, with k-ratios, calculated using intensities from the NIST monte-carlo software (1998). However, the k-ratios utilized here were calculated from the generated x-ray intensities of the compounds and their corresponding pure elements.
Since these are the generated intensities, they are uncorrected for absorption. In addition, the NIST software performs no calculation for characteristic or continuum fluorescence and therefore an essentially ideal generated intensity is calculated.
As we can see, for these compounds, both the mass fraction and electron fraction model give similar errors, as expected, with a possible slight improvement for the electron fraction model. Interestingly enough, you will recall that Te and I are juxtiposed in atomic mass and atomic number, here we note that using the mass fraction we have an error of several percent while the electron fraction prediction error is almost zero. What about compounds containing elements with large differences in A/Z?
Monte-Carlo of Generated Intensities for Compounds with Large differences in A/Z
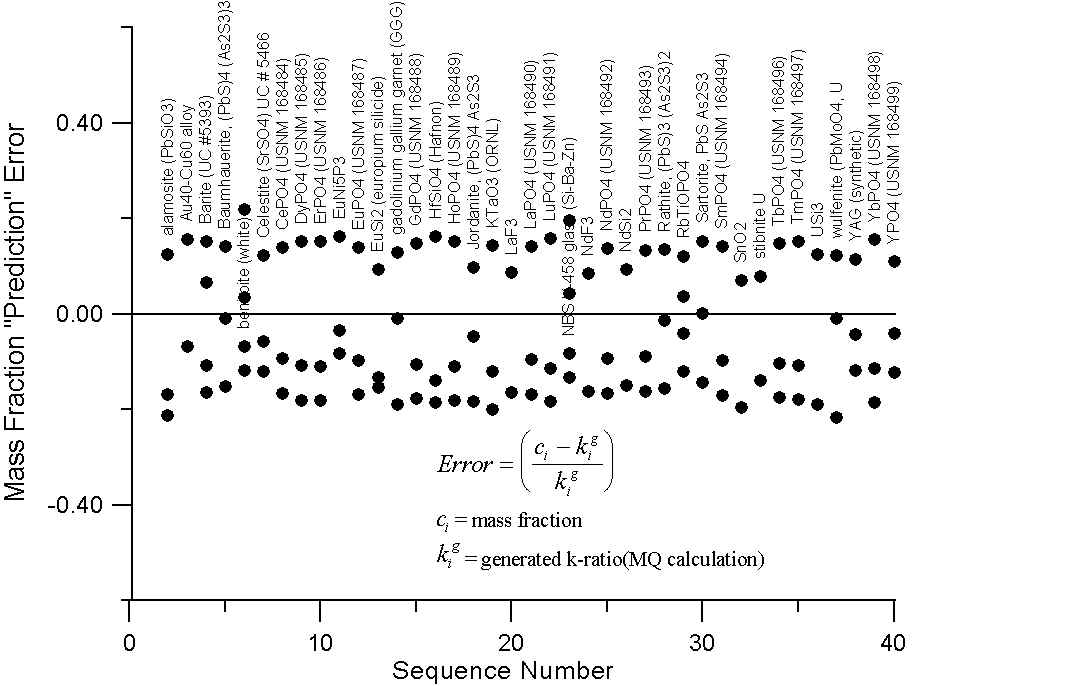
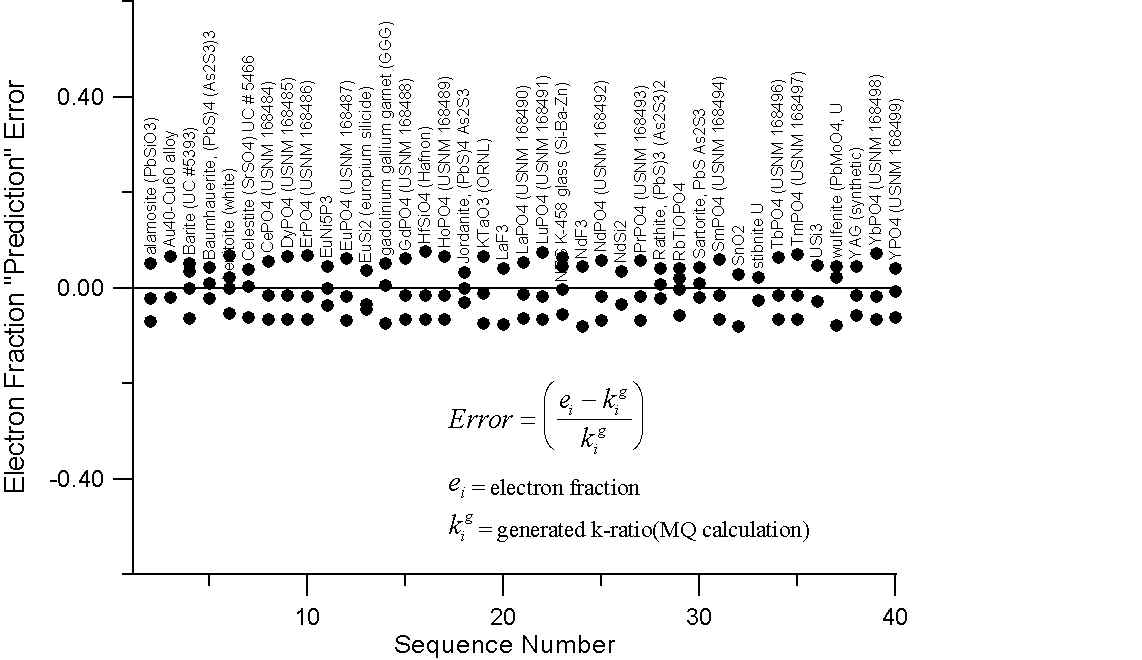
Here we have a graph for those compounds which exhibit A/Z disparities, of at least several percent. The upper plot shows the errors (up to 20%) that we obtain in comparing the mass fraction prediction of Castaing with these "generated" k-ratios.
Compare this to the errors calculated by assuming that the "generated" intensities are equal to the electron fraction of the element in the compounds in the lower plot. Here we can see that the prediction errors are reduced by a factor of two or three using the electron fraction first approximation.
Of course what is missing here is the x-ray intensity lost to backscatter effects. Now, it is our suspicion that the atomic number correction, as it is usually formulated, attempts (at least in part) to compensate for the scaling effect of additional mass in the overall slope of the A/Z curve, due to excess neutrons in high Z elements. Which, as we now know from the isotope data, does not effect electron-solid interactions.
Of course some adjustment is necessary because not all orbital electrons are "equal". That is to say, the more tightly bound inner electrons in higher Z elements do not contribute as much to interactions with incident electrons as do the outer orbital electrons. In a similar way, some of the proton charge of the nucleus is screened by the orbital electrons of high Z elements. Therefore a correction to the electron fraction first approximation will obviously be required to develop a fully quantitative model.
In any event, monte-carlo speculations aside, what empirical evidence can we produce for the electron fraction first approximation? As mentioned before, we can’t directly measure the generated intensities, but we can get close if we select compounds where the absorption, fluorescence and atomic number effects are minimal.
We therefore selected compounds from the NIST k-ratio database for compounds in which the absorption, fluorescence and atomic number effects are each less than 2%. Since the correction effects are small, we might hope that the measured intensities are close to the "ideal" generated intensities. Out of some 1500 binaries only about 180 meet this stringent criteria for minimal matrix correction effects, so the statistics are perhaps not as good as one would like.
In any case, using both the mass and electron fractional models, we calculated the errors by comparing the experimentally measured k-ratios for those compounds with these simple first approximation predictions.
Plot of Mass and Electron Fraction Differences for NIST Intensity Database
(where Absorption, Fluorescence and Atomic Number Effects < 2%)
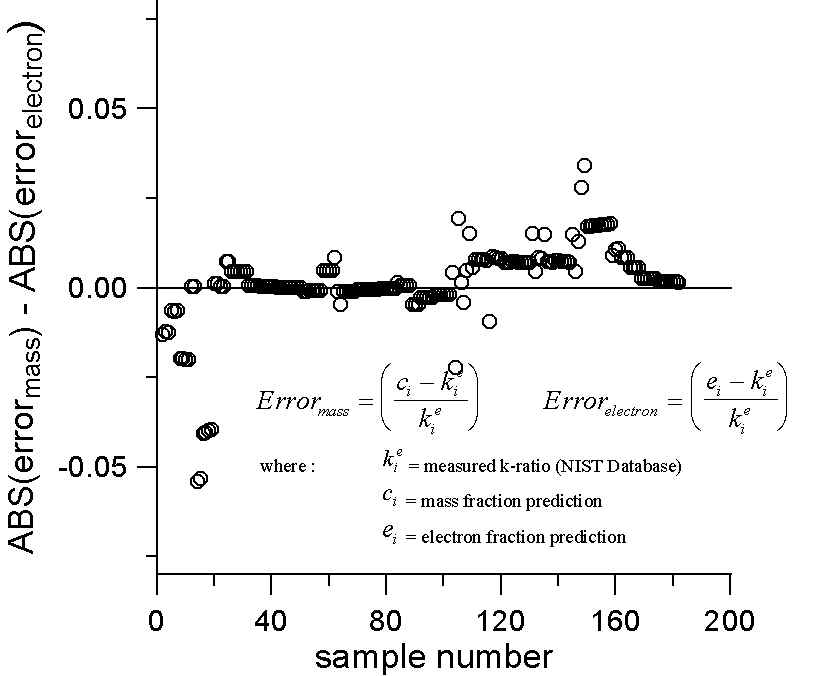
We then plotted the differences in the errors between the two models. A positive value here indicates a larger error for the mass fraction prediction. Although not statistically overwhelming, we can see that in 66% of the cases the mass fraction prediction has larger errors than the electron fraction prediction, when both are compared to the experimental k-ratios. However, it should be noted that these cases were not selected for differences in A/Z, only for their minimal matrix effects.
Now, if we include those binaries from the NIST database that can have a more significant atomic number correction, but still a negligible absorption and fluorescence correction, then we can examine a much larger number of binaries (about 650) and obtain better statistics. Including these binaries in the dataset might be interesting because, as previously stated, it seems to us that current algorithms for the atomic number correction may inadvertently be compensating for the overall slope in the A/Z mass curve of the elements when utilizing mass fractions for scaling.
Plot of Mass and Electron Fraction Differences for NIST Intensity Database
Absorption and Fluorescence Corrections < 2%
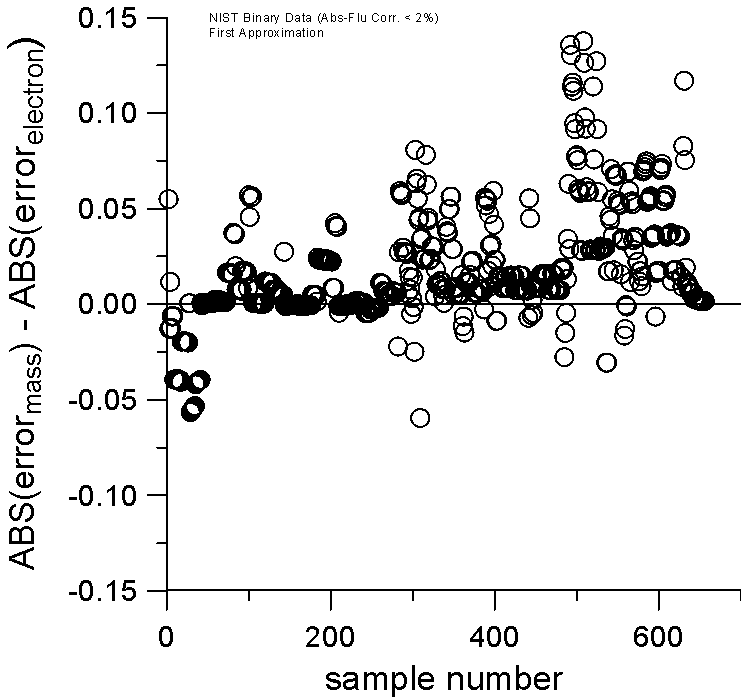
And again, by plotting the relative errors of the two models against each other, by simply subtracting them, it can be seen, even more clearly, that now in over 80% of the cases, the electron fraction first approximation is closer to the experimentally measured k-ratio. Once again, a positive value indicates a larger error for the mass fraction model compared to the electron fraction model.
Summary
- The isotope measurements shown today, clearly refute the idea, that the property we call mass, affects electron-solid interactions for x-ray ionization and electron backscatter productions (at least to typical EPMA precision levels).
- We propose that a first approximation based on electrons is a more physically based model for the interaction ratio of elements in a compound: and that is, electron fractions, the elemental fraction of electrons in a compound:
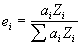
where :
is the atomic fraction of the element i, in the compound
is the atomic number of the element i, in the compound
- To turn the electron fraction first approximation into a fully quantitative model, will undoubtedly require adjustments for nuclear screening effects and differences in orbital binding energies, both of which are strictly a function of Z.
We hope that we have managed to stimulate some interest to take a closer look at some of the assumptions in our field, especially with regard to the use of mass to scale electron-solid interactions. There remains much work to do. What we have presented today is only the first step in developing a new quantitative model for electron probe microanalysis.
We expect that an electron fraction based model should be more simple than current mass based models, since it will not be affected by the arbitrary variation of atomic mass in our "natural abundance averaged" periodic table, which is simply a result of the capricious nature of stellar nucleo-synthesis and nuclear stability processes.
Thank-you.